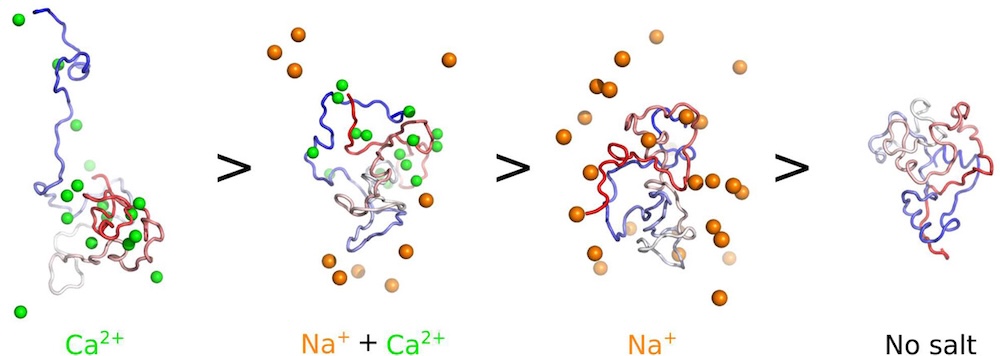
Parkinson’s disease is linked to the aggregation of the intrinsically disordered protein α-synuclein (aSyn), but the precise triggers and mechanisms driving this process remain unclear. Local environmental factors, such as ion concentrations, can influence aSyn’s tendency to aggregate.
A team of researchers including professor Alex Routh have explored how physiologically relevant ions, mainly Ca2+ and Na+, affect aSyn aggregation, monomer structural dynamics, and fibril polymorphism.
In a recent paper published in the Journal of the American Chemical Society, they illustrate the pivotal influence of the local ionic milieu in shaping the structure and aggregation propensity of aSyn, offering insights into the molecular underpinnings of Parkinson’s disease and potential therapeutic strategies targeting aSyn dynamics. You can read the article here.