Catalyst Design for Oxygen Evolution Reactions
Research group: professor Stuart Clarke
The Problem
The oxygen evolution reaction is a vital process for the production of hydrogen from renewable energy, but currently the best known catalysts for the reaction are prohibitively expensive and scarce.
Work Needed
We need to find new catalysts for the reaction that are less costly, less energy intensive, and more sustainable. This is extremely important work to produce hydrogen for the energy transition.
Our Work
We have synthesised a novel class of catalysts that meets these aims. A patent has been filed and we are now testing the commercial feasibility of these promising new catalysts, as well as exploring new related materials.
Background
The oxygen evolution reaction (OER) is a critical part of the chemical process that splits water into hydrogen and oxygen, using renewable energy. The hydrogen is an alternative to fossil fuels that emits no CO2. However, it has proved a significant challenge to develop scalable and cost-effective OER catalysts to make the reaction more efficient. Precious metal oxides are currently understood to be the best OER catalysts, but these are prohibitively expensive and scarce.
Our Work
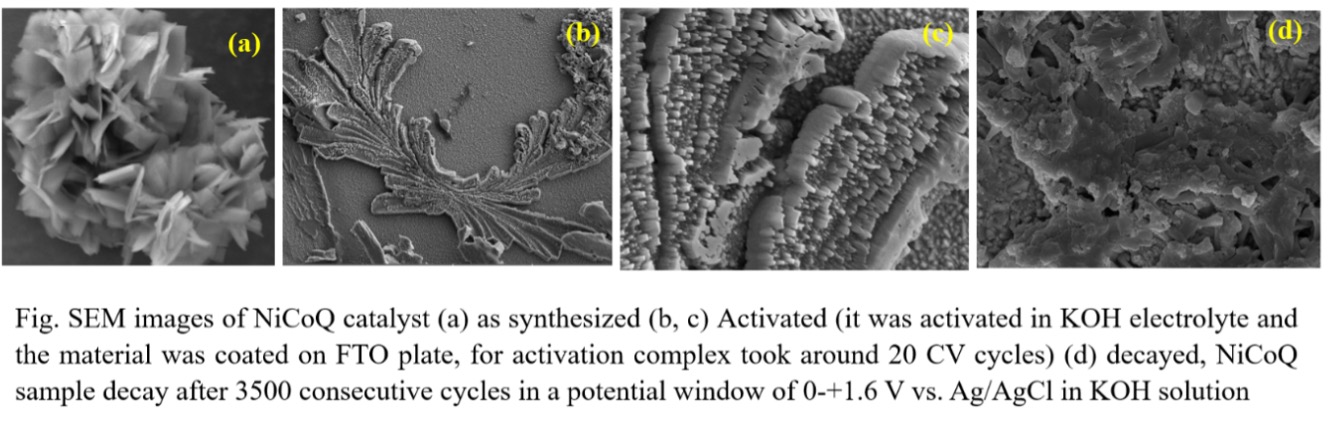
Our team has synthesized a novel class of catalysts to address the limitations of traditional metal catalysts. We employ cheaper transition metals made by a low temperature route, avoiding transition metal oxides and hydroxides which required very high synthesis temperatures (as high as 800°C in some cases), which makes their use energy-intensive and impractical.
We have developed innovative metal coordination complexes consisting of a non-precious metal core and an organic ligand; the ligands make the electronic structure of the complex modifiable, allowing us a controlled way of altering it to optimise the catalysis. Notably, our synthesis process has been carried out at temperatures as low as 80°C, making this method far more efficient and environmentally friendly than others. Our approach also eliminates the need for binders when fabricating electrodes. Unlike conventional methods, our material can be directly coated onto surfaces without requiring additional substances.
Implications
The ability to synthesize cheap, effective OER catalysts at low temperatures and without binders represents a significant step towards more sustainable and cost-effective renewable energy solutions. The materials might also be adopted in other related areas such as hydrogen production, fuel cells, and batteries, where similar reactions are also a key requirement.
Future Directions
We have a dual-pronged approach (1) optimising of the fundamental physical and preparation chemistry and (2) optimising the commercial feasibility of our catalysts, for example testing their performance on commerically relevant substrates. This will help us to find the very best catalyst in this class and evaluate their compatibility and adaptability for industrial applications.