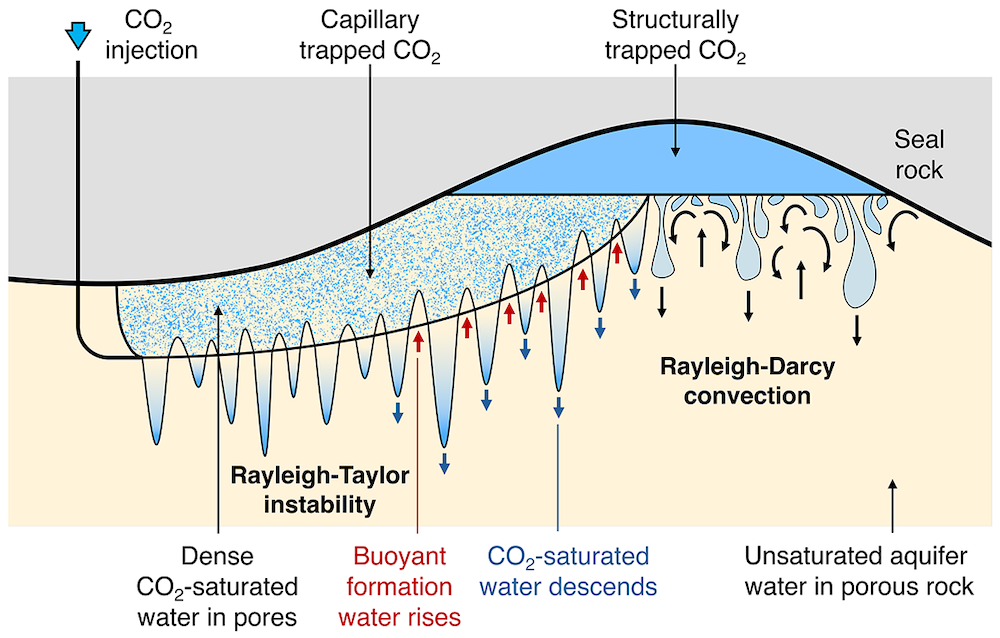
Carbon storage in deep saline aquifers has become an important part of plans to decarbonise the energy system. Typically, aquifers are composed of a porous, permeable rock containing formation water. As CO2 is injected into an aquifer, some of it migrates towards topographic highs within the aquifer, while some is retained in the pore spaces of the rock by capillary forces. This leads to zones of porous rock where individual pore spaces contain capillary-trapped CO2.
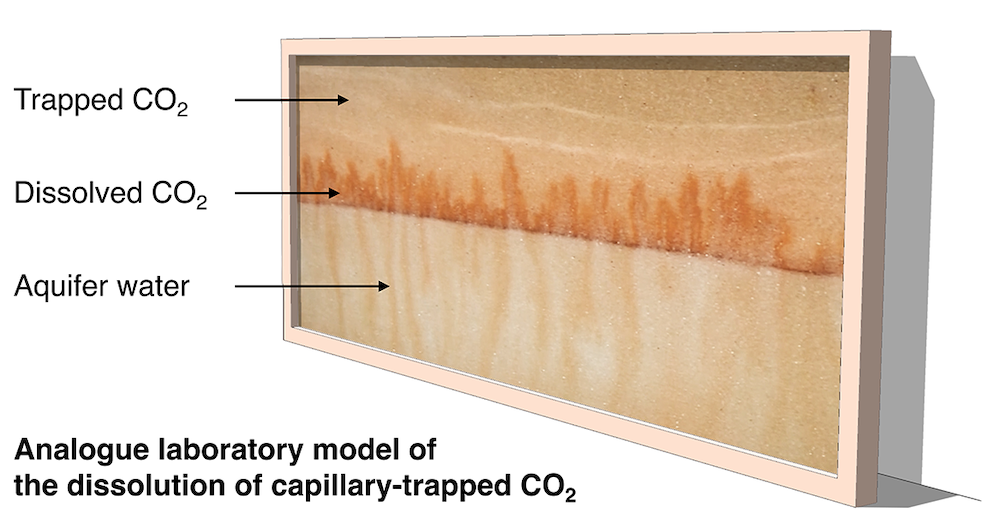
Researchers at the Institute have developed a new laboratory experiment which models the dissolution of the capillary-trapped CO2. The experiment indicates that the pore water surrounding the trapped CO2 rapidly becomes saturated in CO2. Once saturated, the density of this water exceeds that of the background formation water. This leads to convective instability of the water in the aquifer, with fingers of dense, saturated water descending towards the base of the aquifer and fingers of buoyant, unsaturated water rising into the region with trapped CO2. This convective flow controls the dissolution of the capillary-trapped CO2 in the aquifer.
A new model of the finger motion and the non-linear dissolution process predicts the maximum amount of trapped CO2 which can be dissolved in the aquifer, as well as the time required by the dissolution. You can read more about it in a new article published in Communications Earth and Environment, which is available here.